Understanding Total Enthalpy: Definition and Formula Explained
Enthalpy is a thermodynamic property that describes the amount of heat released or absorbed by a system at constant pressure. Total enthalpy, on the other hand, refers to the sum of internal energy and the product of the pressure and volume of a system. In this article, we’ll explore the concept of total enthalpy in detail, including its formula and units, and provide a simple explanation for the confusion it often causes.
The Formula for Total Enthalpy
According to the definition, total enthalpy is given by the equation:
Here, represents the specific enthalpy (enthalpy per unit mass) of a fluid or gas, while represents its velocity. The equation implies that total enthalpy is equal to the sum of the fluid or gas’s specific enthalpy () and half the square of its velocity ().
It’s important to note that the term in the equation is a representation of the kinetic energy of the fluid or gas in motion. The addition of kinetic energy to the total enthalpy equation acknowledges the fact that temperature and pressure changes in a system can be influenced by the motion of the fluid or gas.
The Units of Total Enthalpy
The units of total enthalpy can be a bit confusing since it is expressed as “per unit mass.” In other words, it is the amount of enthalpy per unit mass of fluid or gas in the system. Therefore, the units of total enthalpy would be energy per unit mass, such as joules per kilogram (J/kg).
However, looking at the equation, we can see that the units of the term are velocity squared, or in other words, meters squared per second squared (m^2/s^2). To make both sides of the equation have the same units, the term needs to be multiplied by a conversion factor that relates velocity to energy. This conversion factor is specific to the unit system being used and is called a “dimensional constant.”
For example, in the SI unit system, the conversion factor for velocity to energy is . Therefore, the units of total enthalpy in the SI system would be joules per kilogram (J/kg).
Why Include Kinetic Energy in Total Enthalpy?
At this point, you may be wondering why we need to include kinetic energy in the total enthalpy equation. After all, doesn’t already account for flow work?
The answer is that the inclusion of the term acknowledges the influence of kinetic energy on a fluid or gas’s temperature and pressure changes. In systems where there is significant fluid or gas motion, not accounting for the kinetic energy could result in errors in the calculation of temperature and pressure changes.
By including the kinetic energy term, total enthalpy offers a more accurate representation of the thermodynamic properties of a fluid or gas in motion.
Applications of Total Enthalpy
The concept of total enthalpy is used in a wide range of applications, including the design and analysis of turbines, compressors, and heat exchangers. In these applications, total enthalpy is used to calculate the work output, efficiency, and heat transfer rates of the system.
For example, consider a gas turbine that generates electricity. The combustion of fuel inside the turbine produces a high-pressure gas that expands over turbine blades, producing work that is used to turn a generator. The calculation of the work output and efficiency of the turbine depends on the total enthalpy of the gas at various points in the system.
Conclusion
In summary, total enthalpy is the sum of internal energy and the product of the pressure and volume of a system, and is expressed in energy per unit mass. The inclusion of the term in the total enthalpy equation acknowledges the influence of kinetic energy on a fluid or gas’s temperature and pressure changes. Total enthalpy plays an important role in the design and analysis of various thermodynamic systems, including turbines, compressors, and heat exchangers.
What is Total Enthalpy?
Understanding Total Enthalpy: Definition and Formula Explained
Enthalpy is a thermodynamic property that describes the amount of heat released or absorbed by a system at constant pressure. Total enthalpy, on the other hand, refers to the sum of internal energy and the product of the pressure and volume of a system. In this article, we’ll explore the concept of total enthalpy in detail, including its formula and units, and provide a simple explanation for the confusion it often causes.
The Formula for Total Enthalpy
According to the definition, total enthalpy is given by the equation: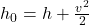
Here,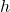 represents the specific enthalpy (enthalpy per unit mass) of a fluid or gas, while
represents the specific enthalpy (enthalpy per unit mass) of a fluid or gas, while  represents its velocity. The equation implies that total enthalpy is equal to the sum of the fluid or gas’s specific enthalpy (
represents its velocity. The equation implies that total enthalpy is equal to the sum of the fluid or gas’s specific enthalpy (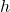 ) and half the square of its velocity (
) and half the square of its velocity (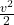 ).
).
It’s important to note that the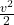 term in the equation is a representation of the kinetic energy of the fluid or gas in motion. The addition of kinetic energy to the total enthalpy equation acknowledges the fact that temperature and pressure changes in a system can be influenced by the motion of the fluid or gas.
term in the equation is a representation of the kinetic energy of the fluid or gas in motion. The addition of kinetic energy to the total enthalpy equation acknowledges the fact that temperature and pressure changes in a system can be influenced by the motion of the fluid or gas.
The Units of Total Enthalpy
The units of total enthalpy can be a bit confusing since it is expressed as “per unit mass.” In other words, it is the amount of enthalpy per unit mass of fluid or gas in the system. Therefore, the units of total enthalpy would be energy per unit mass, such as joules per kilogram (J/kg).
However, looking at the equation, we can see that the units of the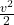 term are velocity squared, or in other words, meters squared per second squared (m^2/s^2). To make both sides of the equation have the same units, the
term are velocity squared, or in other words, meters squared per second squared (m^2/s^2). To make both sides of the equation have the same units, the 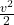 term needs to be multiplied by a conversion factor that relates velocity to energy. This conversion factor is specific to the unit system being used and is called a “dimensional constant.”
term needs to be multiplied by a conversion factor that relates velocity to energy. This conversion factor is specific to the unit system being used and is called a “dimensional constant.”
For example, in the SI unit system, the conversion factor for velocity to energy is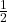 . Therefore, the units of total enthalpy in the SI system would be joules per kilogram (J/kg).
. Therefore, the units of total enthalpy in the SI system would be joules per kilogram (J/kg).
Why Include Kinetic Energy in Total Enthalpy?
At this point, you may be wondering why we need to include kinetic energy in the total enthalpy equation. After all, doesn’t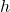 already account for flow work?
already account for flow work?
The answer is that the inclusion of the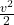 term acknowledges the influence of kinetic energy on a fluid or gas’s temperature and pressure changes. In systems where there is significant fluid or gas motion, not accounting for the kinetic energy could result in errors in the calculation of temperature and pressure changes.
term acknowledges the influence of kinetic energy on a fluid or gas’s temperature and pressure changes. In systems where there is significant fluid or gas motion, not accounting for the kinetic energy could result in errors in the calculation of temperature and pressure changes.
By including the kinetic energy term, total enthalpy offers a more accurate representation of the thermodynamic properties of a fluid or gas in motion.
Applications of Total Enthalpy
The concept of total enthalpy is used in a wide range of applications, including the design and analysis of turbines, compressors, and heat exchangers. In these applications, total enthalpy is used to calculate the work output, efficiency, and heat transfer rates of the system.
For example, consider a gas turbine that generates electricity. The combustion of fuel inside the turbine produces a high-pressure gas that expands over turbine blades, producing work that is used to turn a generator. The calculation of the work output and efficiency of the turbine depends on the total enthalpy of the gas at various points in the system.
Conclusion
In summary, total enthalpy is the sum of internal energy and the product of the pressure and volume of a system, and is expressed in energy per unit mass. The inclusion of the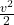 term in the total enthalpy equation acknowledges the influence of kinetic energy on a fluid or gas’s temperature and pressure changes. Total enthalpy plays an important role in the design and analysis of various thermodynamic systems, including turbines, compressors, and heat exchangers.
term in the total enthalpy equation acknowledges the influence of kinetic energy on a fluid or gas’s temperature and pressure changes. Total enthalpy plays an important role in the design and analysis of various thermodynamic systems, including turbines, compressors, and heat exchangers.