In this article, we will discuss the process of numerical simulation and calculations of pH. We will start by discussing the chemical reactions involved in the process and how they affect the pH of the solution.
Chemical Reactions
The first step in the process is the reaction of addition in water. This is a simple reaction where reacts with water to form carbonic acid ().
+
The next step is to increase the pH of the solution to 12 by adding 1M . The reaction in this step would be:
+ +
The final step is to add the new solution at pH 12 to solution. The final reaction of the system would be:
( + + ) + + +
Calculations
Now, we can move on to the calculations. The first question we need to address is whether the final chemical reaction is correct or not. The final reaction appears to be correct because it follows the law of mass conservation.
In the second question, we are asked to calculate the pH of the solution by setting the pH of step c equal to 12, and adding 50 ml of that to solution. In order to do the calculations, we need to know the concentrations of the different ions in the solution.
The concentration of ions can be calculated using the formula:
Since the pH is 12, the concentration of ions would be:
The concentration of ions can be calculated using the formula:
Since we know the concentration of ions, the concentration of ions can be calculated as:
Finally, the concentration of ions is assumed to be 0.05 M in 50 ml.
Using these concentrations, we can calculate the final pH of the solution using the equation:
Substituting the values, we get:
This confirms that the final pH of the solution is indeed 12.
Numerical Simulation
Finally, we need to address the question of whether it is possible to do a numerical simulation of the change in the overall solution pH, or predict the final pH by calculations. The answer is yes.
We can use a software like MATLAB to simulate the change in the pH of the solution over time, based on the initial concentrations of the different ions and the different chemical reactions involved.
We can also use various mathematical models to predict the final pH of the solution by doing calculations based on the concentrations of the different ions and the different chemical reactions involved. These predictions can then be compared to the actual experimental results to validate the accuracy of the models.
Conclusion
In conclusion, numerical simulation and calculations of pH are important tools in chemical research and analysis. By understanding the chemical reactions involved, and using appropriate mathematical models and software, we can accurately predict the behavior of chemical systems and design new experiments to test our predictions.
Numerical Simulation And Calculations of Ph
In this article, we will discuss the process of numerical simulation and calculations of pH. We will start by discussing the chemical reactions involved in the process and how they affect the pH of the solution.
Chemical Reactions
The first step in the process is the reaction of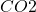 addition in water. This is a simple reaction where
addition in water. This is a simple reaction where 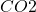 reacts with water to form carbonic acid (
reacts with water to form carbonic acid (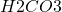 ).
).
The next step is to increase the pH of the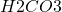 solution to 12 by adding 1M
solution to 12 by adding 1M 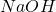 . The reaction in this step would be:
. The reaction in this step would be:
The final step is to add the new solution at pH 12 to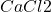 solution. The final reaction of the system would be:
solution. The final reaction of the system would be:
(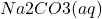 +
+ 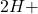 +
+ 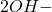 ) +
) + 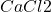
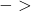
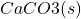 +
+ 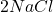 +
+ 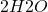
Calculations
Now, we can move on to the calculations. The first question we need to address is whether the final chemical reaction is correct or not. The final reaction appears to be correct because it follows the law of mass conservation.
In the second question, we are asked to calculate the pH of the solution by setting the pH of step c equal to 12, and adding 50 ml of that to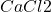 solution. In order to do the calculations, we need to know the concentrations of the different ions in the solution.
solution. In order to do the calculations, we need to know the concentrations of the different ions in the solution.
The concentration of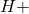 ions can be calculated using the formula:
ions can be calculated using the formula:
Since the pH is 12, the concentration of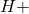 ions would be:
ions would be:
The concentration of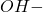 ions can be calculated using the formula:
ions can be calculated using the formula:
Since we know the concentration of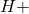 ions, the concentration of
ions, the concentration of 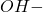 ions can be calculated as:
ions can be calculated as:
Finally, the concentration of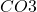 ions is assumed to be 0.05 M in 50 ml.
ions is assumed to be 0.05 M in 50 ml.
Using these concentrations, we can calculate the final pH of the solution using the equation:
Substituting the values, we get:
This confirms that the final pH of the solution is indeed 12.
Numerical Simulation
Finally, we need to address the question of whether it is possible to do a numerical simulation of the change in the overall solution pH, or predict the final pH by calculations. The answer is yes.
We can use a software like MATLAB to simulate the change in the pH of the solution over time, based on the initial concentrations of the different ions and the different chemical reactions involved.
We can also use various mathematical models to predict the final pH of the solution by doing calculations based on the concentrations of the different ions and the different chemical reactions involved. These predictions can then be compared to the actual experimental results to validate the accuracy of the models.
Conclusion
In conclusion, numerical simulation and calculations of pH are important tools in chemical research and analysis. By understanding the chemical reactions involved, and using appropriate mathematical models and software, we can accurately predict the behavior of chemical systems and design new experiments to test our predictions.