Measuring Change in Concentration of Hydrogen Peroxide
If you are planning on conducting an experiment to find the rate constant of the decomposition of hydrogen peroxide, you will need to measure the change in concentration over time. In this article, we will explore several methods that can be used to measure the change in concentration of hydrogen peroxide.
Method 1: Titration
Titration is a common laboratory technique used to determine the concentration of a solution. In this method, a known concentration of a reagent is added slowly to the solution being tested until the reaction is complete. The volume of the reagent required to complete the reaction is recorded, and using stoichiometry, the concentration of the original solution can be calculated.
To measure the concentration of hydrogen peroxide using titration, a known concentration of a reagent, such as potassium permanganate (KMnO4), can be slowly added to the solution. The reaction between potassium permanganate and hydrogen peroxide can be represented by the following equation:
In this reaction, the purple color of potassium permanganate solution disappears as it reacts with hydrogen peroxide. The point at which all of the hydrogen peroxide has reacted can be determined by the disappearance of the purple color.
Once the reaction is complete, the volume of the potassium permanganate solution required can be recorded, and using stoichiometry, the concentration of hydrogen peroxide can be calculated.
Method 2: Spectrophotometry
Spectrophotometry is a technique used to measure the concentration of a chemical in a solution by measuring the amount of light absorbed by the solution. In this method, a known concentration of a reagent is added to the solution, and the absorbance of the resulting solution is measured at a specific wavelength using a spectrophotometer. The concentration of the original solution can be calculated using Beer’s law.
To measure the concentration of hydrogen peroxide using spectrophotometry, a known concentration of a reagent, such as potassium permanganate, can be added to the solution. The absorbance of the resulting solution can be measured at a specific wavelength, such as 240 nm. Using Beer’s law, the concentration of hydrogen peroxide can be calculated.
Beer’s law can be represented as follows:
Where,
A is the absorbance of a solution
is the molar extinction coefficient, which is specific for each chemical and wavelength
l is the path length of the solution, which is the length of the sample cell or cuvette
c is the concentration of the solution
Method 3: Gas Volume Measurements
Another method to measure the change in concentration of hydrogen peroxide is through gas volume measurements. When hydrogen peroxide decomposes, oxygen gas is produced, and the amount of oxygen produced is directly proportional to the amount of hydrogen peroxide decomposed.
To measure the amount of oxygen produced, the gas can be collected in a gas syringe or a gas burette. The volume of oxygen produced can be measured, and using stoichiometry, the amount of hydrogen peroxide decomposed can be calculated.
The reaction between hydrogen peroxide and manganese dioxide, which is commonly used as a catalyst, can be represented by the following equation:
In this reaction, the amount of oxygen produced is directly proportional to the amount of hydrogen peroxide decomposed.
Conclusion
Measuring the change in concentration of hydrogen peroxide is essential in determining the rate constant of its decomposition. Several methods, such as titration, spectrophotometry, and gas volume measurements, can be used to measure the change in concentration. Each of these methods has its advantages and disadvantages, and the choice of method will depend on the resources available and the precision required.
Regardless of the method chosen, it is essential to ensure that the measurements are accurate and precise to obtain reliable results.
Measuring Change In Concentration of Hydrogen Peroxide
Measuring Change in Concentration of Hydrogen Peroxide
If you are planning on conducting an experiment to find the rate constant of the decomposition of hydrogen peroxide,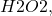 you will need to measure the change in concentration over time. In this article, we will explore several methods that can be used to measure the change in concentration of hydrogen peroxide.
you will need to measure the change in concentration over time. In this article, we will explore several methods that can be used to measure the change in concentration of hydrogen peroxide.
Method 1: Titration
Titration is a common laboratory technique used to determine the concentration of a solution. In this method, a known concentration of a reagent is added slowly to the solution being tested until the reaction is complete. The volume of the reagent required to complete the reaction is recorded, and using stoichiometry, the concentration of the original solution can be calculated.
To measure the concentration of hydrogen peroxide using titration, a known concentration of a reagent, such as potassium permanganate (KMnO4), can be slowly added to the solution. The reaction between potassium permanganate and hydrogen peroxide can be represented by the following equation:
In this reaction, the purple color of potassium permanganate solution disappears as it reacts with hydrogen peroxide. The point at which all of the hydrogen peroxide has reacted can be determined by the disappearance of the purple color.
Once the reaction is complete, the volume of the potassium permanganate solution required can be recorded, and using stoichiometry, the concentration of hydrogen peroxide can be calculated.
Method 2: Spectrophotometry
Spectrophotometry is a technique used to measure the concentration of a chemical in a solution by measuring the amount of light absorbed by the solution. In this method, a known concentration of a reagent is added to the solution, and the absorbance of the resulting solution is measured at a specific wavelength using a spectrophotometer. The concentration of the original solution can be calculated using Beer’s law.
To measure the concentration of hydrogen peroxide using spectrophotometry, a known concentration of a reagent, such as potassium permanganate, can be added to the solution. The absorbance of the resulting solution can be measured at a specific wavelength, such as 240 nm. Using Beer’s law, the concentration of hydrogen peroxide can be calculated.
Beer’s law can be represented as follows:
Where,
Method 3: Gas Volume Measurements
Another method to measure the change in concentration of hydrogen peroxide is through gas volume measurements. When hydrogen peroxide decomposes, oxygen gas is produced, and the amount of oxygen produced is directly proportional to the amount of hydrogen peroxide decomposed.
To measure the amount of oxygen produced, the gas can be collected in a gas syringe or a gas burette. The volume of oxygen produced can be measured, and using stoichiometry, the amount of hydrogen peroxide decomposed can be calculated.
The reaction between hydrogen peroxide and manganese dioxide, which is commonly used as a catalyst, can be represented by the following equation:
In this reaction, the amount of oxygen produced is directly proportional to the amount of hydrogen peroxide decomposed.
Conclusion
Measuring the change in concentration of hydrogen peroxide is essential in determining the rate constant of its decomposition. Several methods, such as titration, spectrophotometry, and gas volume measurements, can be used to measure the change in concentration. Each of these methods has its advantages and disadvantages, and the choice of method will depend on the resources available and the precision required.
Regardless of the method chosen, it is essential to ensure that the measurements are accurate and precise to obtain reliable results.