Metal carbonyls are compounds composed of a metal atom bonded to carbon monoxide ligands. The vibrational frequencies of the carbon monoxide ( ) bonds in these compounds can be used to determine certain properties of the compounds. In this article, we will discuss the trend of -vibration in isoelectronic metal carbonyls and why the atomic charge does not always determine the trend.
The Trend of Co-Vibration in Metal Carbonyls
The table below shows the vibrational frequencies of the bonds in several isoelectronic metal carbonyls.
These complexes are isoelectronic, meaning they have the same number of electrons. The trend is expected to lie in the atomic charge . A higher atomic charge should lead to more metal-ligand attraction, which should lead to higher electron density, more -backbonding, weaker bonds, and lower wavenumber. However, this is not the case here.
Explaining the Trend
Instead of the atomic charge, the trend of the -vibration in metal carbonyls is determined by the energy level of the Highest Occupied Molecular Orbital (HOMO).
In metal carbonyls, the ligands donate electrons to the metal center while also accepting electrons back from it. This phenomenon is known as backbonding, and it is a result of the interaction between the filled -orbitals of and the empty d-orbitals of the metal. The strength of this interaction determines the energy level of the HOMO.
As we move from nickel to titanium, the HOMO energy level decreases. The titanium compound has the lowest HOMO energy level, which leads to the highest wavenumber of -vibration. This is because the increased energy level of the HOMO leads to a stronger backbonding interaction. As a result, the bond becomes stronger, and the wavenumber increases.
Implications of the Trend
The trend of -vibration in metal carbonyls has important implications. For example, it can help chemists determine the metal-ligand bonding in a compound. By analyzing the -vibration frequencies, we can determine the strength of the backbonding interaction and infer the relative strengths of the metal-ligand interactions.
Conclusion
In conclusion, the trend of -vibration in metal carbonyls is not determined by the atomic charge but by the energy level of the HOMO. The strength of the backbonding interaction determines the wavenumber of -vibration, and this has important implications for understanding the metal-ligand bonding in these compounds.
Co-vibration In Metal Carbonyl
Understanding Co-Vibration in Metal Carbonyls
Metal carbonyls are compounds composed of a metal atom bonded to carbon monoxide ligands. The vibrational frequencies of the carbon monoxide (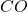 ) bonds in these compounds can be used to determine certain properties of the compounds. In this article, we will discuss the trend of
) bonds in these compounds can be used to determine certain properties of the compounds. In this article, we will discuss the trend of 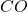 -vibration in isoelectronic metal carbonyls and why the atomic charge does not always determine the trend.
-vibration in isoelectronic metal carbonyls and why the atomic charge does not always determine the trend.
The Trend of Co-Vibration in Metal Carbonyls
The table below shows the vibrational frequencies of the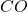 bonds in several isoelectronic metal carbonyls.
bonds in several isoelectronic metal carbonyls.
These complexes are isoelectronic, meaning they have the same number of electrons. The trend is expected to lie in the atomic charge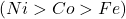 . A higher atomic charge should lead to more metal-ligand attraction, which should lead to higher electron density, more
. A higher atomic charge should lead to more metal-ligand attraction, which should lead to higher electron density, more 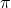 -backbonding, weaker
-backbonding, weaker 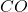 bonds, and lower wavenumber. However, this is not the case here.
bonds, and lower wavenumber. However, this is not the case here.
Explaining the Trend
Instead of the atomic charge, the trend of the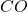 -vibration in metal carbonyls is determined by the energy level of the Highest Occupied Molecular Orbital (HOMO).
-vibration in metal carbonyls is determined by the energy level of the Highest Occupied Molecular Orbital (HOMO).
In metal carbonyls, the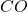 ligands donate electrons to the metal center while also accepting electrons back from it. This phenomenon is known as backbonding, and it is a result of the interaction between the filled
ligands donate electrons to the metal center while also accepting electrons back from it. This phenomenon is known as backbonding, and it is a result of the interaction between the filled 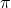 -orbitals of
-orbitals of 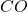 and the empty d-orbitals of the metal. The strength of this interaction determines the energy level of the HOMO.
and the empty d-orbitals of the metal. The strength of this interaction determines the energy level of the HOMO.
As we move from nickel to titanium, the HOMO energy level decreases. The titanium compound has the lowest HOMO energy level, which leads to the highest wavenumber of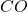 -vibration. This is because the increased energy level of the HOMO leads to a stronger backbonding interaction. As a result, the
-vibration. This is because the increased energy level of the HOMO leads to a stronger backbonding interaction. As a result, the 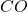 bond becomes stronger, and the wavenumber increases.
bond becomes stronger, and the wavenumber increases.
Implications of the Trend
The trend of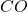 -vibration in metal carbonyls has important implications. For example, it can help chemists determine the metal-ligand bonding in a compound. By analyzing the
-vibration in metal carbonyls has important implications. For example, it can help chemists determine the metal-ligand bonding in a compound. By analyzing the 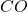 -vibration frequencies, we can determine the strength of the backbonding interaction and infer the relative strengths of the metal-ligand interactions.
-vibration frequencies, we can determine the strength of the backbonding interaction and infer the relative strengths of the metal-ligand interactions.
Conclusion
In conclusion, the trend of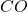 -vibration in metal carbonyls is not determined by the atomic charge but by the energy level of the HOMO. The strength of the backbonding interaction determines the wavenumber of
-vibration in metal carbonyls is not determined by the atomic charge but by the energy level of the HOMO. The strength of the backbonding interaction determines the wavenumber of 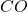 -vibration, and this has important implications for understanding the metal-ligand bonding in these compounds.
-vibration, and this has important implications for understanding the metal-ligand bonding in these compounds.