Distilling off ether from a mixture of ethyl ether (), ethyl alcohol (), and water can be quite tricky. You want to ensure that minimal water is distilled off as the boiling point of water is 100 °C, while the solvent has a temperature that is approximately 20 °C lower. One way to achieve this is by using a water bath to maintain a consistent temperature. However, is it safe and reasonable to substitute a water bath with a 95% ethyl alcohol bath to prevent substantial evaporation without needing to closely keep track of the temperature?
Alcohol Bath: A Possible Solution?
In theory, an alcohol bath can be used as a substitute for a water bath as it has a boiling point of approximately 78 °C, which is lower than water, thus allowing for a more precise temperature regulation. Additionally, an alcohol bath has lower surface tension than water, which is advantageous for distillation.
However, there are some considerations to keep in mind before using an alcohol bath.
Points to Consider Before Using an Alcohol Bath
Firstly, alcohol is flammable and has a low flash point, which means that it can ignite easily. Therefore, all ignition sources should be removed from the distillation area. Additionally, safety precautions must be taken, such as wearing safety goggles and using a fume hood to ensure that the fumes are whisked away from the laboratory.
Secondly, any impurities such as water or dust can cause ethanol to burn, so care needs to be taken to ensure that the alcohol used is pure and there are no impurities in the distillation flask or bath.
Temperature Regulation in an Alcohol Bath
One of the primary advantages of using an alcohol bath is that it is easier to regulate temperature, particularly as the boiling point of 95% ethanol is commonly known to be 78.4 °C. Therefore, you can adjust the temperature of your alcohol bath to be slightly lower than the boiling point of your solvent (in this case, ethyl ether) to ensure that minimal amounts of water are distilled off too. Additionally, ethanol has a high specific heat capacity, which means that it can take a lot of energy before it starts boiling, allowing for more precise temperature control.
However, it is essential to keep in mind that the temperature of the bath will rise as the distillation proceeds, so adjustments must be made to prevent a temperature that is too high, as this can affect the accuracy of your results. Therefore, the use of temperature-control systems such as water-cooled condensate, heating mantle or a simple thermometer should be used to monitor the temperature of the alcohol bath.
Conclusion
Though using an alcohol bath as a substitute for a water bath may seem advantageous, it should only be done with caution. Care must be taken to ensure that the alcohol used is pure, and safety precautions must be adhered to to prevent any burns or accidental ignition. However, if temperature regulation is your concern, an alcohol bath might be the perfect solution for you.
Can You Heat Something at Alcohol Bath?
Distilling off ether from a mixture of ethyl ether (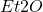 ), ethyl alcohol (
), ethyl alcohol (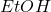 ), and water can be quite tricky. You want to ensure that minimal water is distilled off as the boiling point of water is 100 °C, while the solvent has a temperature that is approximately 20 °C lower. One way to achieve this is by using a water bath to maintain a consistent temperature. However, is it safe and reasonable to substitute a water bath with a 95% ethyl alcohol bath to prevent substantial
), and water can be quite tricky. You want to ensure that minimal water is distilled off as the boiling point of water is 100 °C, while the solvent has a temperature that is approximately 20 °C lower. One way to achieve this is by using a water bath to maintain a consistent temperature. However, is it safe and reasonable to substitute a water bath with a 95% ethyl alcohol bath to prevent substantial 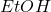 evaporation without needing to closely keep track of the temperature?
evaporation without needing to closely keep track of the temperature?
Alcohol Bath: A Possible Solution?
In theory, an alcohol bath can be used as a substitute for a water bath as it has a boiling point of approximately 78 °C, which is lower than water, thus allowing for a more precise temperature regulation. Additionally, an alcohol bath has lower surface tension than water, which is advantageous for distillation.
However, there are some considerations to keep in mind before using an alcohol bath.
Points to Consider Before Using an Alcohol Bath
Firstly, alcohol is flammable and has a low flash point, which means that it can ignite easily. Therefore, all ignition sources should be removed from the distillation area. Additionally, safety precautions must be taken, such as wearing safety goggles and using a fume hood to ensure that the fumes are whisked away from the laboratory.
Secondly, any impurities such as water or dust can cause ethanol to burn, so care needs to be taken to ensure that the alcohol used is pure and there are no impurities in the distillation flask or bath.
Temperature Regulation in an Alcohol Bath
One of the primary advantages of using an alcohol bath is that it is easier to regulate temperature, particularly as the boiling point of 95% ethanol is commonly known to be 78.4 °C. Therefore, you can adjust the temperature of your alcohol bath to be slightly lower than the boiling point of your solvent (in this case, ethyl ether) to ensure that minimal amounts of water are distilled off too. Additionally, ethanol has a high specific heat capacity, which means that it can take a lot of energy before it starts boiling, allowing for more precise temperature control.
However, it is essential to keep in mind that the temperature of the bath will rise as the distillation proceeds, so adjustments must be made to prevent a temperature that is too high, as this can affect the accuracy of your results. Therefore, the use of temperature-control systems such as water-cooled condensate, heating mantle or a simple thermometer should be used to monitor the temperature of the alcohol bath.
Conclusion
Though using an alcohol bath as a substitute for a water bath may seem advantageous, it should only be done with caution. Care must be taken to ensure that the alcohol used is pure, and safety precautions must be adhered to to prevent any burns or accidental ignition. However, if temperature regulation is your concern, an alcohol bath might be the perfect solution for you.