When it comes to the acidic cleavage of ether with a 2° alkyl group, the mechanism involved is quite different from the ones seen in ethers with 3° alkyl group and 1° alkyl group or two 1° alkyl groups.
Reaction Mechanism
The acidic cleavage of ether with a 2° alkyl group involves the use of a halogen acid (HX), typically hydrochloric acid (HCl) or hydrobromic acid (HBr), which acts as a proton source. The mechanism is slightly similar to the acidic hydrolysis of esters.
Here’s how the reaction mechanism works:
1. The halogen acid protonates the ether to form an oxonium ion.
2. The oxygen atom in the oxonium ion acts as a nucleophile and attacks the 2° carbon.
3. This results in the formation of a carbocation intermediate and an alcohol molecule.
4. The halide ion (X-) acts as a nucleophile and attacks the carbocation, leading to the formation of a 2° alkyl halide and a protonated alcohol.
It’s worth noting that this reaction mechanism involves both and reactions, which means that the product will depend on the reaction conditions.
Products Obtained
When an ether with a 2° alkyl group and a 1° alkyl group is cleaved with a halogen acid, two products are obtained:
A 2° alkyl halide
A protonated alcohol
The protonated alcohol that’s obtained can undergo further dehydration to form an alkene. However, this step is reversible and depends on the reaction conditions.
Examples
Let’s take a look at some examples to better understand the acidic cleavage of ether with a 2° alkyl group:
Example 1:
CH3CH2CH(OCH2CH3)CH3 + HBr → CH3CH2CHBrCH3 + HOCH2CH3
Example 2:
CH3(CH2)3CH(OCH3)CH3 + HCl → CH3(CH2)3CHClCH3 + HOCH3
In example 1, the ether is propyl ethyl ether, and the product that’s obtained is 2-bromopropane and ethanol. In example 2, the ether is butyl methyl ether, and the product that’s obtained is 1-chlorobutane and methanol.
Conclusion
So there you have it, the acidic cleavage of ether with a 2° alkyl group involves the use of a halogen acid, which results in the formation of a 2° alkyl halide and a protonated alcohol. This reaction mechanism involves both and reactions and is reversible depending on the reaction conditions.
Acidic Cleavage of Ether With a 2° Alkyl Group
When it comes to the acidic cleavage of ether with a 2° alkyl group, the mechanism involved is quite different from the ones seen in ethers with 3° alkyl group and 1° alkyl group or two 1° alkyl groups.
Reaction Mechanism
The acidic cleavage of ether with a 2° alkyl group involves the use of a halogen acid (HX), typically hydrochloric acid (HCl) or hydrobromic acid (HBr), which acts as a proton source. The mechanism is slightly similar to the acidic hydrolysis of esters.
Here’s how the reaction mechanism works:
It’s worth noting that this reaction mechanism involves both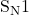 and
and 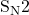 reactions, which means that the product will depend on the reaction conditions.
reactions, which means that the product will depend on the reaction conditions.
Products Obtained
When an ether with a 2° alkyl group and a 1° alkyl group is cleaved with a halogen acid, two products are obtained:
The protonated alcohol that’s obtained can undergo further dehydration to form an alkene. However, this step is reversible and depends on the reaction conditions.
Examples
Let’s take a look at some examples to better understand the acidic cleavage of ether with a 2° alkyl group:
In example 1, the ether is propyl ethyl ether, and the product that’s obtained is 2-bromopropane and ethanol. In example 2, the ether is butyl methyl ether, and the product that’s obtained is 1-chlorobutane and methanol.
Conclusion
So there you have it, the acidic cleavage of ether with a 2° alkyl group involves the use of a halogen acid, which results in the formation of a 2° alkyl halide and a protonated alcohol. This reaction mechanism involves both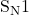 and
and 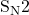 reactions and is reversible depending on the reaction conditions.
reactions and is reversible depending on the reaction conditions.