Before delving into the answer to this question, we need to first understand what resonance structures are and how they relate to the stability of molecules.
What Are Resonance Structures?
Resonance structures, also known as resonant structures or mesomers, are a way to represent the delocalization of electrons within a molecule. In other words, they are a way to show that the electrons in a molecule are not confined to a single bond or atom, but are instead shared among multiple positions.
Resonance structures are typically drawn using a combination of double-headed arrows and dotted lines to represent the sharing of electrons. For example, the resonance structure of the nitrate ion (NO3–) is shown below:
O
// \
O--N--O-
\\ /
O
Here, the double-headed arrows indicate that the electrons in the nitrogen-oxygen bonds are shared between both the nitrogen and both of the oxygen atoms. This sharing of electrons results in a molecule that is more stable than if the electrons were confined to single bonds.
Why Are Resonance Structures Important?
Resonance structures are important for several reasons:
They help explain why certain molecules are more stable than others.
They help explain the reactivity of certain molecules.
They are often used in organic chemistry to predict the behavior of molecules.
Which Compound Has Resonance Structures?
Now that we understand what resonance structures are and why they are important, let’s revisit the original question: which compound has resonance structures?
The answer, as given in the answer key, is D:
But why does have resonance structures?
The answer lies in the structure of the carbonate ion. The carbonate ion is composed of one carbon atom and three oxygen atoms, with a total charge of -2:
O
// \
O--C--O2-
\\ /
O
Each of the oxygen atoms is connected to the carbon atom through a double bond, but these double bonds are not fixed in place. Instead, the electrons are delocalized across all four atoms, resulting in two resonance structures:
O O
// \ / \
O--C==O O==C--O
\\ / \ /
O O
These two resonance structures represent the fact that the electrons in the carbonate ion are not confined to a single position, but are shared among all three oxygen atoms and the carbon atom. This sharing of electrons results in a molecule that is more stable than if the electrons were confined to single bonds.
Conclusion
Resonance structures are an important concept in chemistry, particularly in organic chemistry. They help explain the stability and reactivity of molecules, and are often used to predict the behavior of organic compounds.
While many compounds can have resonance structures, the example given in the original question – – is a particularly good example, as it demonstrates how resonance structures can occur even in ionic compounds.
Which Compound Has Resonance Structures?
Before delving into the answer to this question, we need to first understand what resonance structures are and how they relate to the stability of molecules.
What Are Resonance Structures?
Resonance structures, also known as resonant structures or mesomers, are a way to represent the delocalization of electrons within a molecule. In other words, they are a way to show that the electrons in a molecule are not confined to a single bond or atom, but are instead shared among multiple positions.
Resonance structures are typically drawn using a combination of double-headed arrows and dotted lines to represent the sharing of electrons. For example, the resonance structure of the nitrate ion (NO3–) is shown below:
O // \ O--N--O- \\ / OHere, the double-headed arrows indicate that the electrons in the nitrogen-oxygen bonds are shared between both the nitrogen and both of the oxygen atoms. This sharing of electrons results in a molecule that is more stable than if the electrons were confined to single bonds.
Why Are Resonance Structures Important?
Resonance structures are important for several reasons:
Which Compound Has Resonance Structures?
Now that we understand what resonance structures are and why they are important, let’s revisit the original question: which compound has resonance structures?
The answer, as given in the answer key, is D: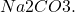
But why does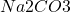 have resonance structures?
have resonance structures?
The answer lies in the structure of the carbonate ion. The carbonate ion is composed of one carbon atom and three oxygen atoms, with a total charge of -2:
O // \ O--C--O2- \\ / OEach of the oxygen atoms is connected to the carbon atom through a double bond, but these double bonds are not fixed in place. Instead, the electrons are delocalized across all four atoms, resulting in two resonance structures:
O O // \ / \ O--C==O O==C--O \\ / \ / O OThese two resonance structures represent the fact that the electrons in the carbonate ion are not confined to a single position, but are shared among all three oxygen atoms and the carbon atom. This sharing of electrons results in a molecule that is more stable than if the electrons were confined to single bonds.
Conclusion
Resonance structures are an important concept in chemistry, particularly in organic chemistry. They help explain the stability and reactivity of molecules, and are often used to predict the behavior of organic compounds.
While many compounds can have resonance structures, the example given in the original question –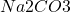 – is a particularly good example, as it demonstrates how resonance structures can occur even in ionic compounds.
– is a particularly good example, as it demonstrates how resonance structures can occur even in ionic compounds.